-
Stability and shelf-life
-
Safety and efficacy
-
Delivery and bioavailability
-
Container compatibility
Labcorp operates its formulation development workflows to quality by design (QbD) principles as outlined in ICH Q8 (R2) (Pharmaceutical Development) and ICH Q9 (Quality Risk Management), with the initial production of a quality target product profile (QTPP) to identify the desired target product formulation attributes.
We recommend that formulation development is performed early, in line with process development, and to minimize subsequent process and analytical validation issues. However, formulation development is often a protracted process as increased understanding of the product warrants modifications.
Our formulation services
Pre-formulation
Forced degradation where little is known about the routes of degradation

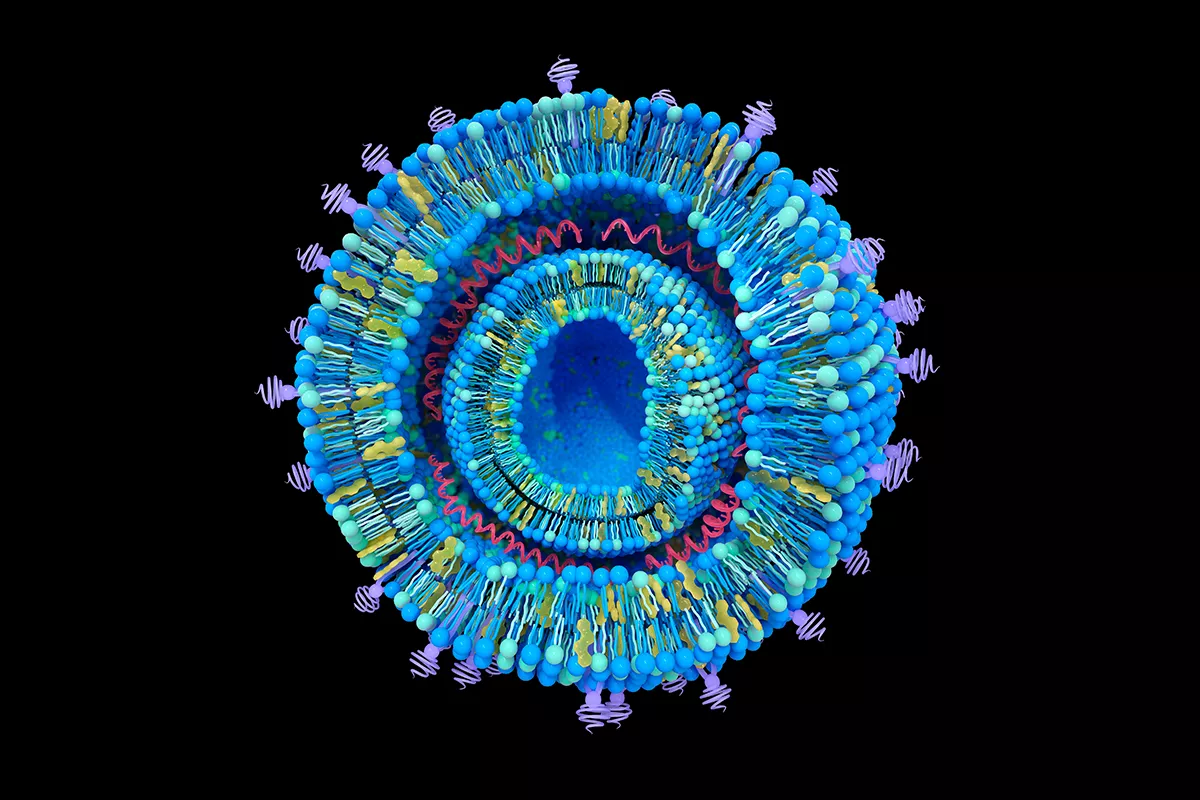
Nucleic acid-LNP using microfluidics
mRNA and other nucleic acid therapeutics encapsulated in lipid nanoparticles (LNPs)
Monoclonal antibodies (mAbs) and antibody-related products
A focused formulation development workflow to minimize the analytical load for antibody therapies