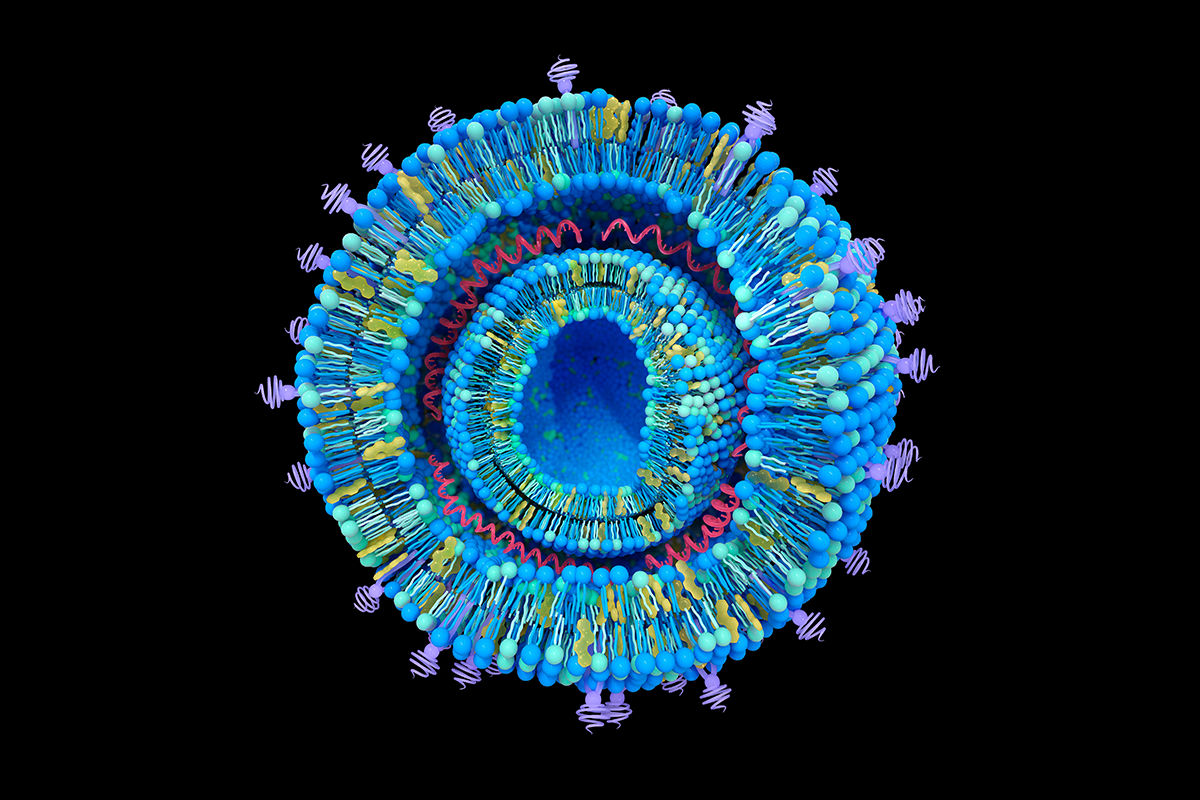
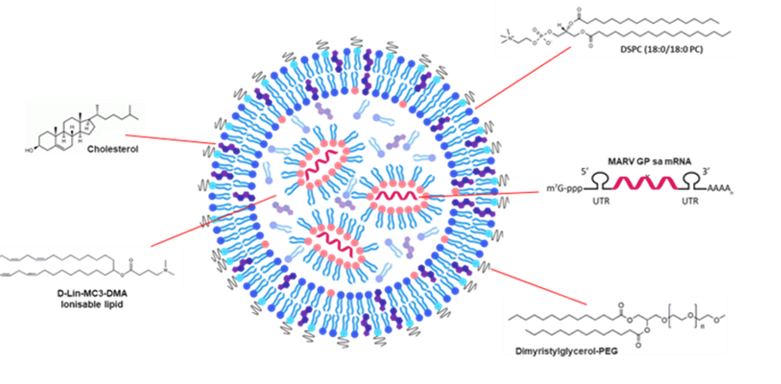
Ionizable lipids
Charge-neutral at physiological pH but become positively charged at acidic pH, enabling mRNA binding and endosomal escape
Phospholipids
Stabilize the lipid bilayer
Cholesterol
Enhances membrane stability and fluidity
PEGylated lipids
Provide a hydrophilic “stealth” coating to improve circulation time and prevent immune recognition
Additional actives
Agents to modulate activity or cell-specific delivery
Optimal LNP formulation development also requires the optimization of the microfluidic process
-
Preparation of mRNA and lipid solutions: selection of the pH and ionic strength of the aqueous buffer which will impact the charge and stability of the mRNA and lipids, providing optimal electrostatic interaction for encapsulation
-
Mixing parameters involving mixer design, flow rate ratio (FRR: ratio of aqueous to ethanolic solution) and total flow rate (TFR)
-
Buffer exchange by dialysis/ultrafiltration to remove solvent and exchange the LNPs into a physiological buffer and sterile filtration
In line with ICH Q8 (R2)(Pharmaceutical Development), ICH Q9(Quality Risk Management), i.e., QbD, Labcorp biopharmaceutical CMC services’ LNP formulation development process incorporates a design of experiment (DoE) statistical approach to evaluate both LNP components and the microfluidic process. Using DoE provides the maximum amount of data for the minimum number of samples and hence also reduces the analytical load. The outcome of these studies is a lead formulation that will meet prescribed critical quality attributes, such as particle size and size distribution, zeta potential, % mRNA encapsulation and mRNA integrity. Real-time, accelerated and forced degradation studies can be applied to determine the formulation provides sufficient stability and expected shelf life.