Personalized biomarker support to help drive your development from bench to commercialization
Biomarkers are at the epicenter of today’s drug development and the pursuit of personalized medicines, which accounted for more than 40% of FDA approvals in 2018.1 They help define disease biology, drive patient identification and stratification, identify mechanism of action and understand the pharmacodynamic aspects of a drug, among other aspects.
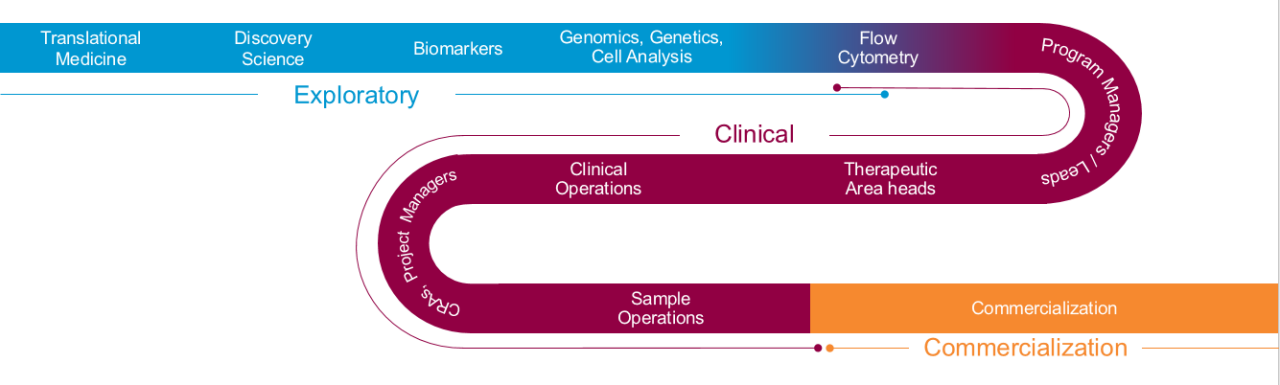
A clear path forward for efficient drug development
To fully realize the benefits of a biomarker strategy, you need early insights, focused expertise and a clear blueprint that includes the most advanced techniques and platforms. It begins with a deep understanding of the role of biomarkers in your program, the proposed application, and which tools will help you achieve your goals.
Selecting the most appropriate regulatory environment for your biomarker program must also be considered. Rapid development, validation and—when needed—transfer of your assay will optimize your timeline and financial impact.
1Personalized Medicine Coalition. Personalized Medicine at FDA: A Progress & Outlook Report, 2018
An approach as unique as your molecule
Led by our Biomarker Solution Center team of PhDs focused on key therapeutic areas, the design for your biomarker program is informed by industry insights and deep specialized knowledge around three key areas:

)