What is Prostate Health Index (phi)?
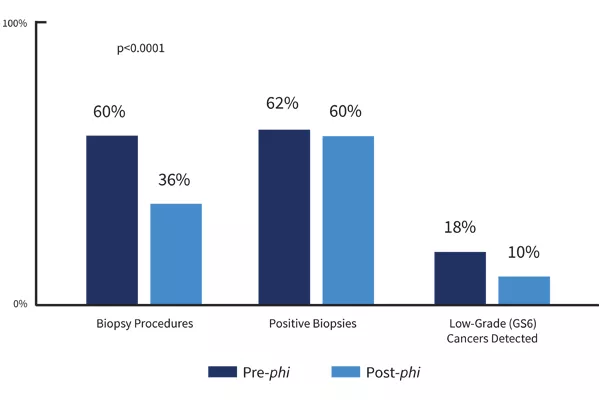
The Prostate Health Index (phi) is a prostate cancer risk assessment that improves early detection of prostate cancer and can help reduce unnecessary, negative prostate biopsies. Although prostate biopsies are a common procedure, they do carry the risks associated with any invasive medical procedure, and they can also be uncomfortable for the patient. phi incorporates the results of three blood tests—PSA, free PSA and p2PSA—to calculate a phi score. The phi score provides key information about the likelihood that patients with elevated PSA have cancer, as opposed to other, more benign conditions that can also cause elevated PSA scores.

)