More than 90% of all pancreatic cancers are classified as ductal adenocarcinomas and, within the western-world, pancreatic cancer is the fourth leading cause of cancer related deaths.
Prognosis with pancreatic cancer is extremely poor, with a 5-year relative survival rate of 5% and median survival of 3.5 months for patients with Stage III non-resectable tumors.1 Unfortunately, the incidence of pancreatic cancer has been on the rise while the 5-year survival rate has not changed. Surgical resection is the only potentially curative therapy, but only 10% of patients are diagnosed early enough for this to be an option and most who are eligible for surgery ultimately relapse. As with many other types of cancer, pancreatic cancer grows silently for years without any symptoms. In most cases diagnosis is not made until the cancer has grown outside of the pancreas to other proximal tissues and/or has metastasized. These patients are left with very few meaningful options. Therefore, effective novel therapies are sorely needed in treatment of pancreatic cancer.
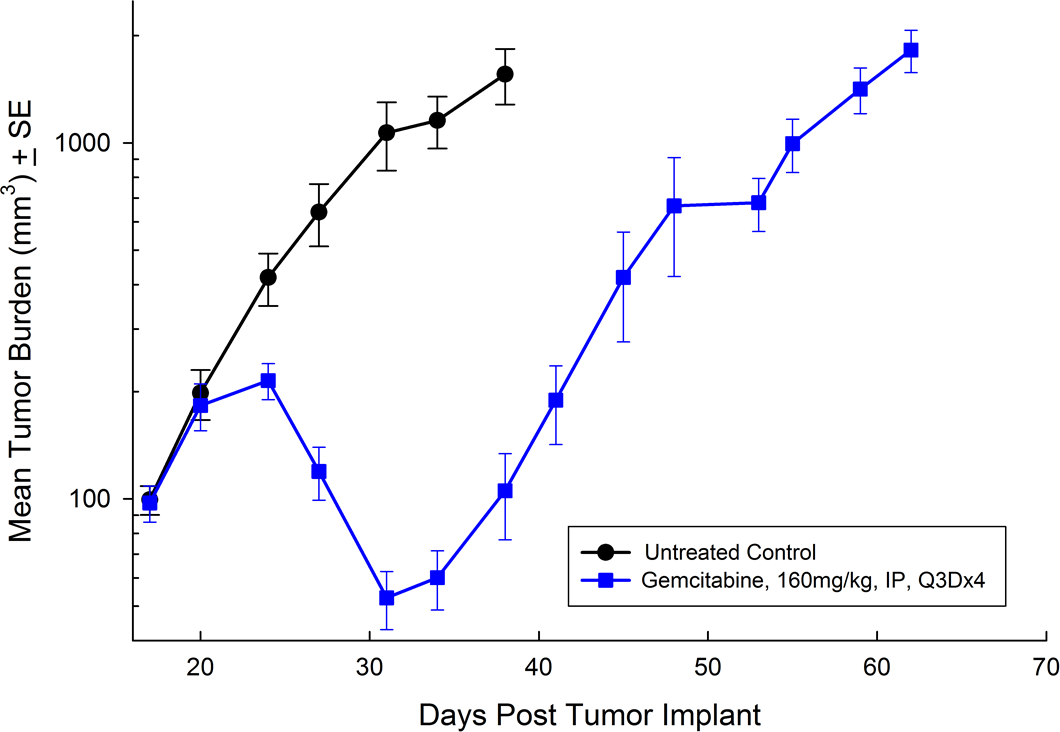
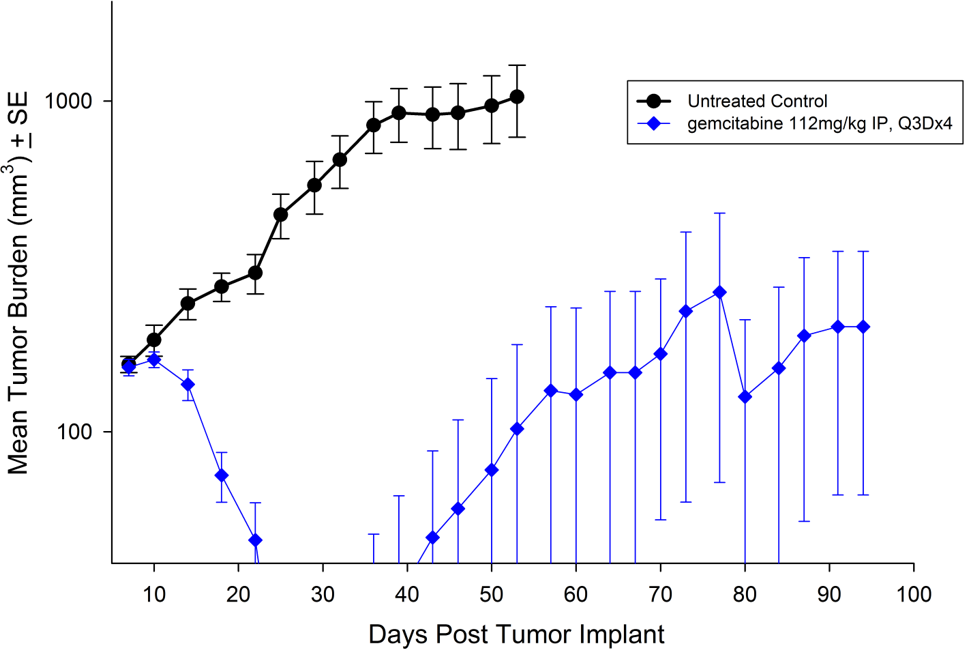
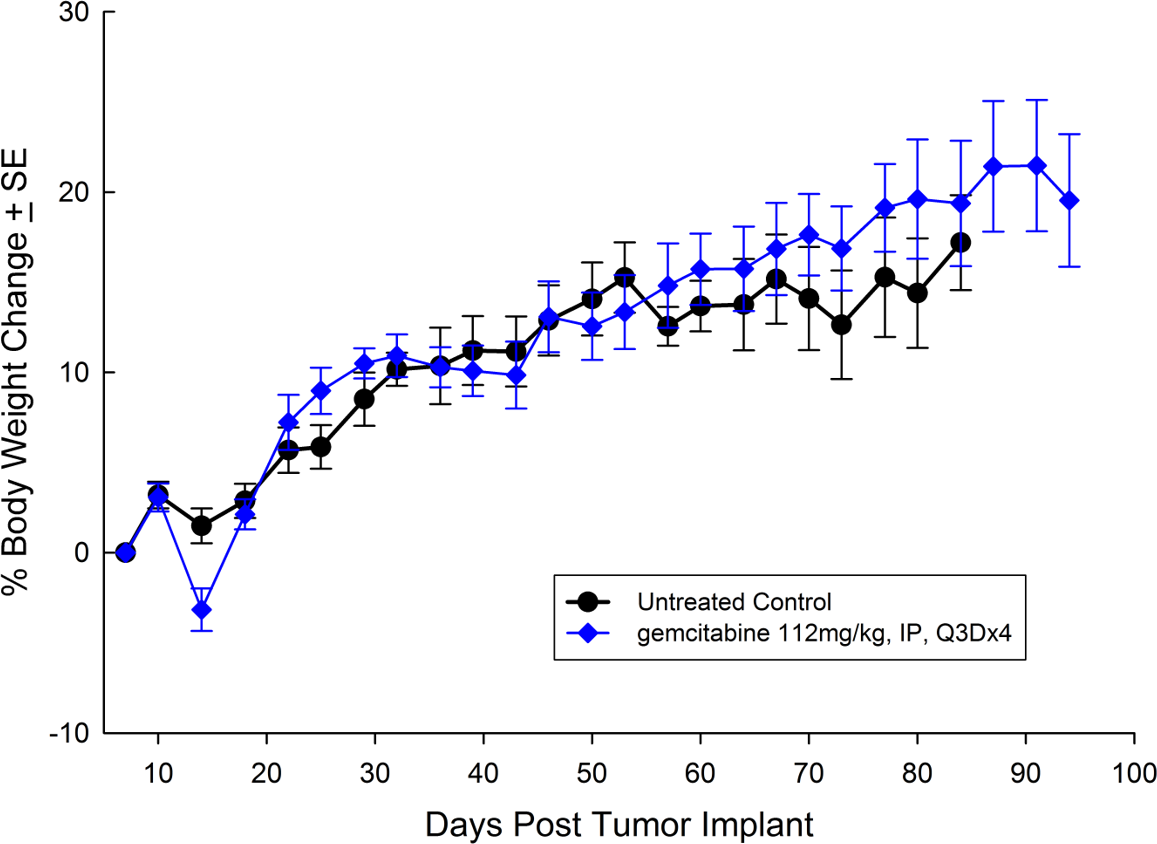
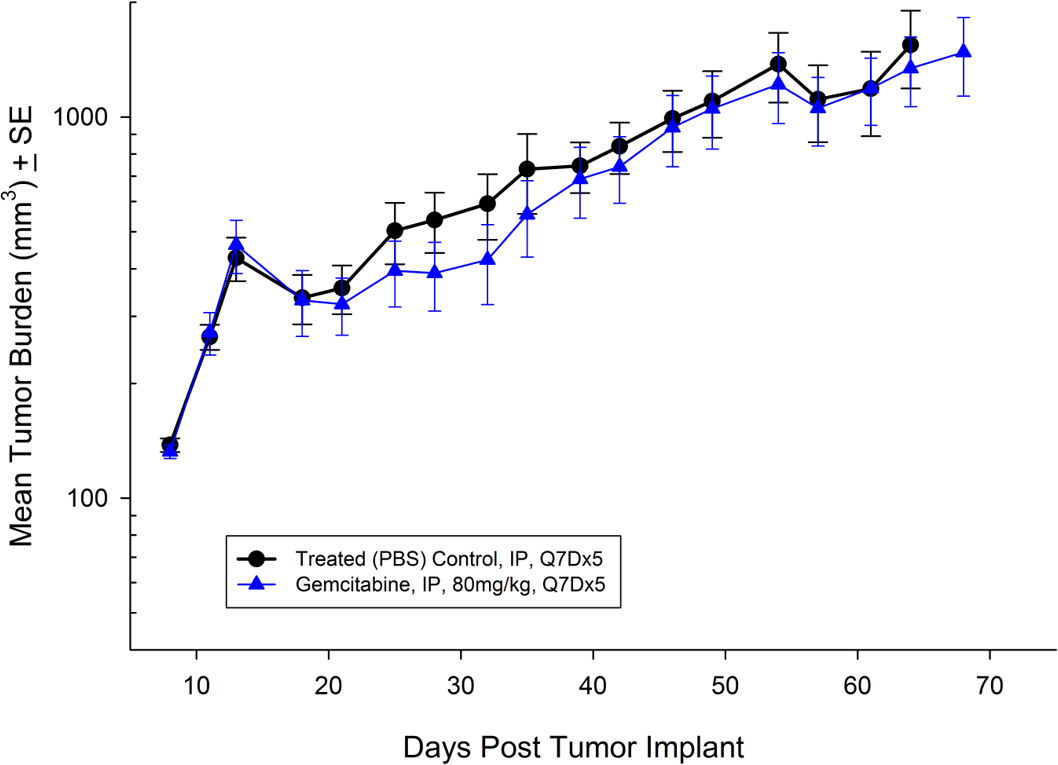
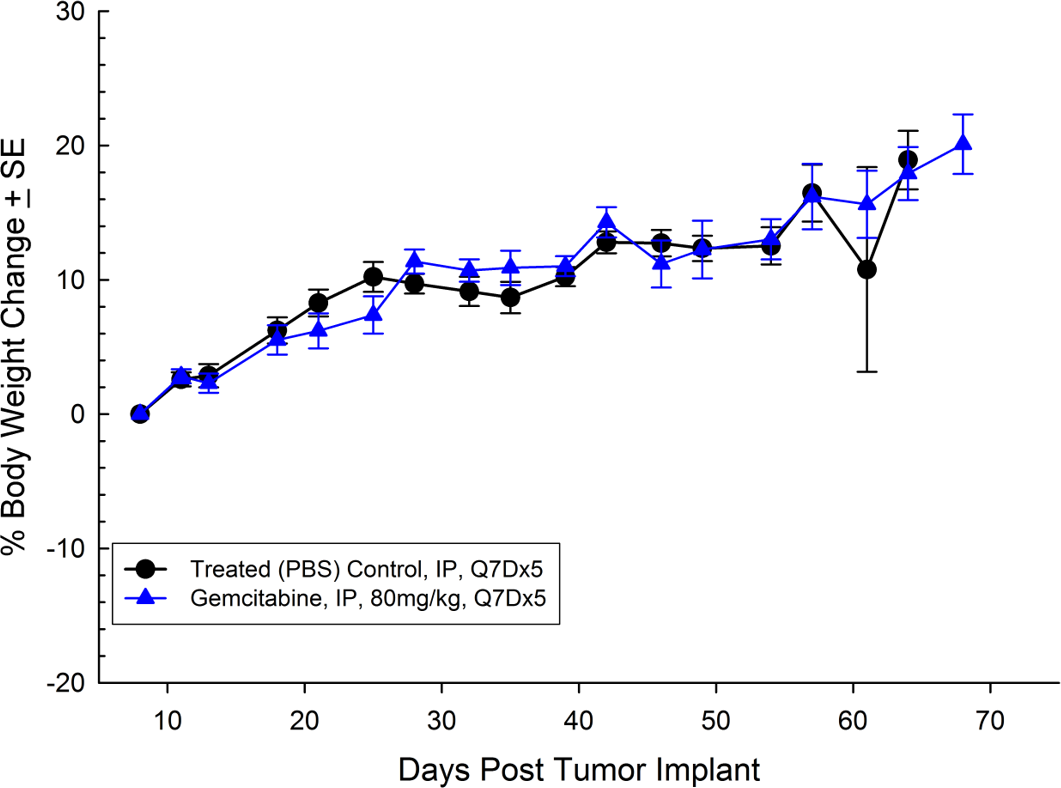
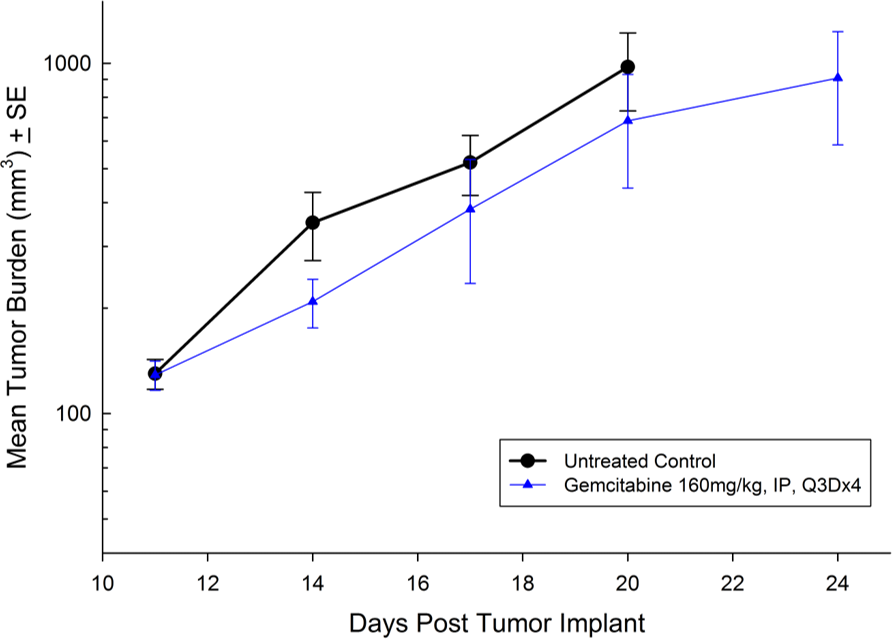
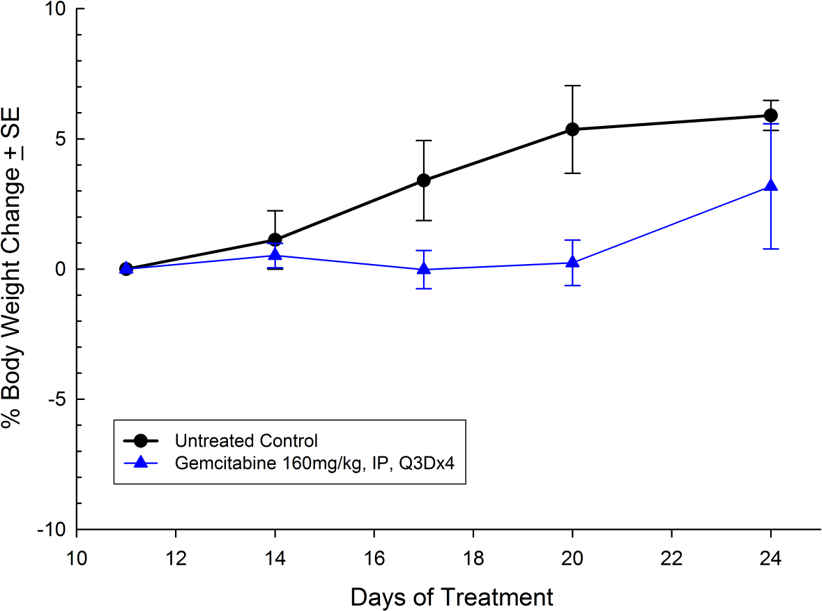
For the last 15 years, patients diagnosed with advanced stage pancreatic cancer are given gemcitabine (Gemzar®) as the standard first line treatment. Preclinically, we use gemcitabine as our standard of care to provide a benchmark to our clients looking to surpass current clinical treatment options or to combine with novel therapies; such as targeted agents and immune-modulators.
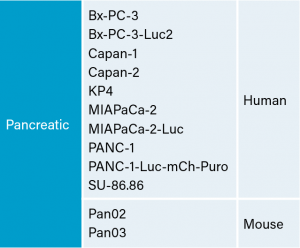
There are several human and murine pancreatic cell lines available to the preclinical cancer research community to aid in the development of novel therapies. Labcorp has a large panel of pancreatic lines ready for testing (see Table 1). We have optimized and characterized the subcutaneous (SC) growth for several of these models and evaluated their response to gemcitabine treatment.