01 Apr 2019
Author: Erin Trachet | Director, Scientific Development
Date: April 2019
Bladder cancer is one of the most frequent cancers of the urinary tract, accounting for about 80,000 new cases and 18,000 deaths in the United States in 2018, according to the National Cancer Society. Typically, patients with bladder cancer have limited surgical or treatment options. Traditional chemotherapeutics are ineffective, and surgery is often used to diagnose bladder cancer and to determine whether the cancer has spread into (invaded) the muscle layer of the bladder wall. When bladder cancer is invasive, all or part of the bladder may need to be removed, leaving the patient with long term adverse effects. In an effort to meet the need for advances in bladder cancer treatment, in 2018 the FDA accelerated the approvals for two checkpoint inhibitors, Keytruda and Tecentriq.
The steady growth, and interest in immunotherapy has required continual development and optimization of syngeneic mouse tumor models with desirable growth kinetics and response to immunomodulatory agents. One of these bladder cancer models, MB49, has been characterized by Labcorp to support development of these agents. MB49 cells (urothelial carcinoma) were derived from a C57BL/6 mouse following exposure of primary bladder cells to DMBA (7,12-dimethylbenz[a]anthracene) for 24 hours followed by culturing.[1]
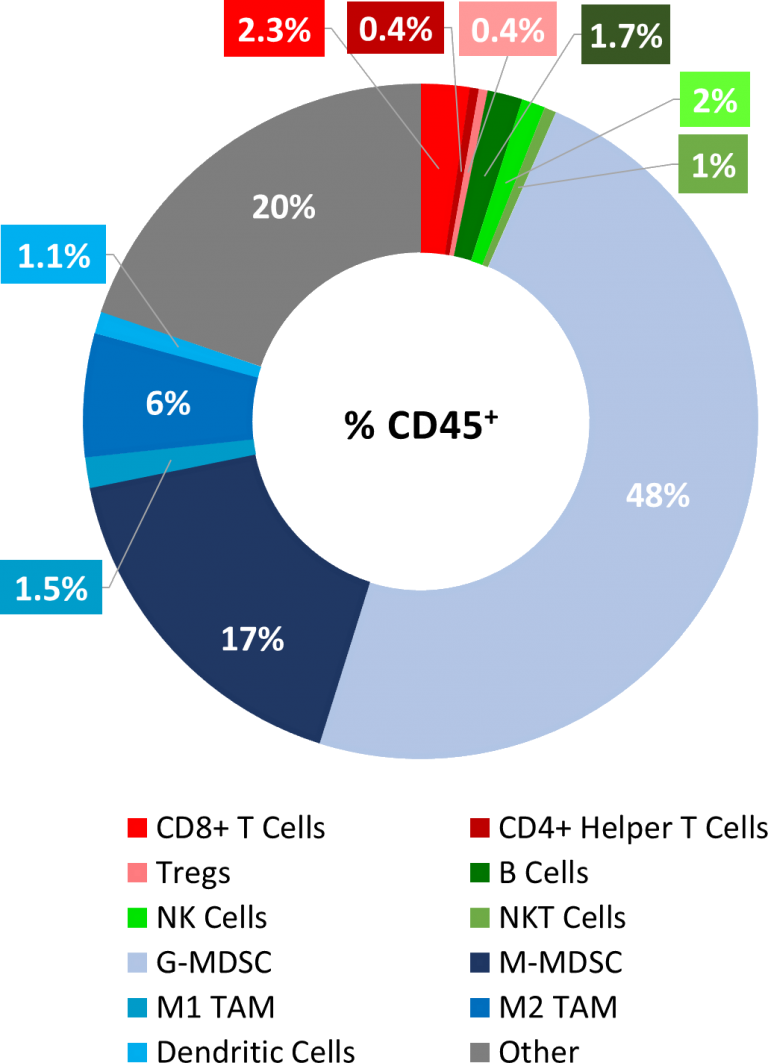
MB49 Baseline Tumor Immune Profile
As described below, MB49 is considered to have a cold tumor phenotype with very low levels of CD8+ and CD4+ T cell infiltration (<3%) and significant presence of immunosuppressive myeloid populations (~65%), see Figure 1. The preliminary immunomodulatory treatment data shows mild responses, further supporting a cold tumor phenotype, and indicating that this model has the potential to respond to immune stimulants, perhaps best in combination with other agents. Thus, MB49 is well positioned to be a powerful immuno-oncology model with significant utility in drug development.
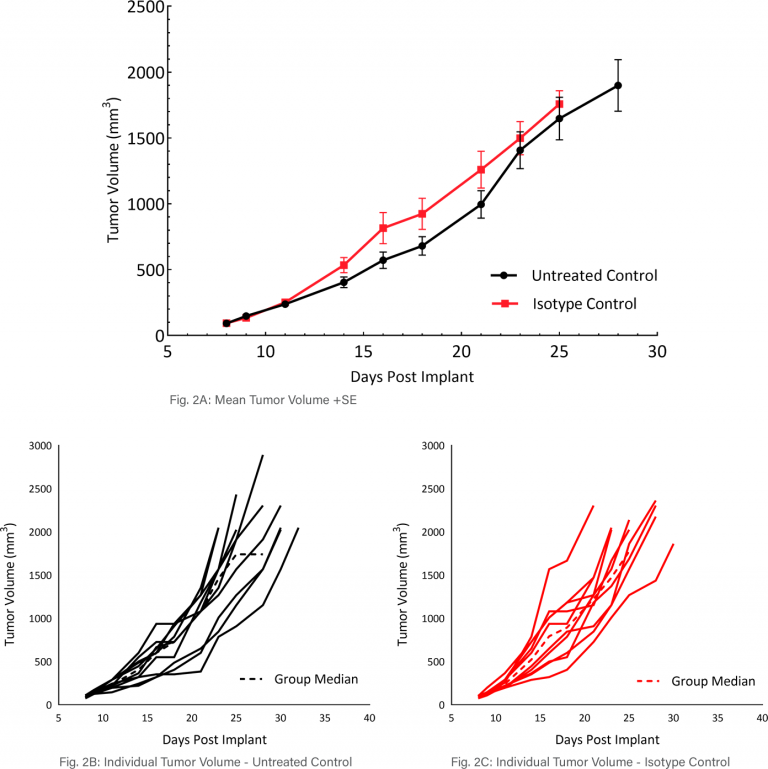
Mean and Individual Growth of MB49 Tumors
The in vivo doubling time of subcutaneous MB49 tumors is approximately four days, a moderate growth rate which can facilitate up to a three-week dosing window for test agents to elicit their anti-tumor activity (Figure 2A). Figure 2 demonstrates mean tumor volumes (Fig 2A) and individual tumor volumes of untreated control tumors (Fig 2B) compared to those treated with isotype control (Fig 2C). No differences were observed.
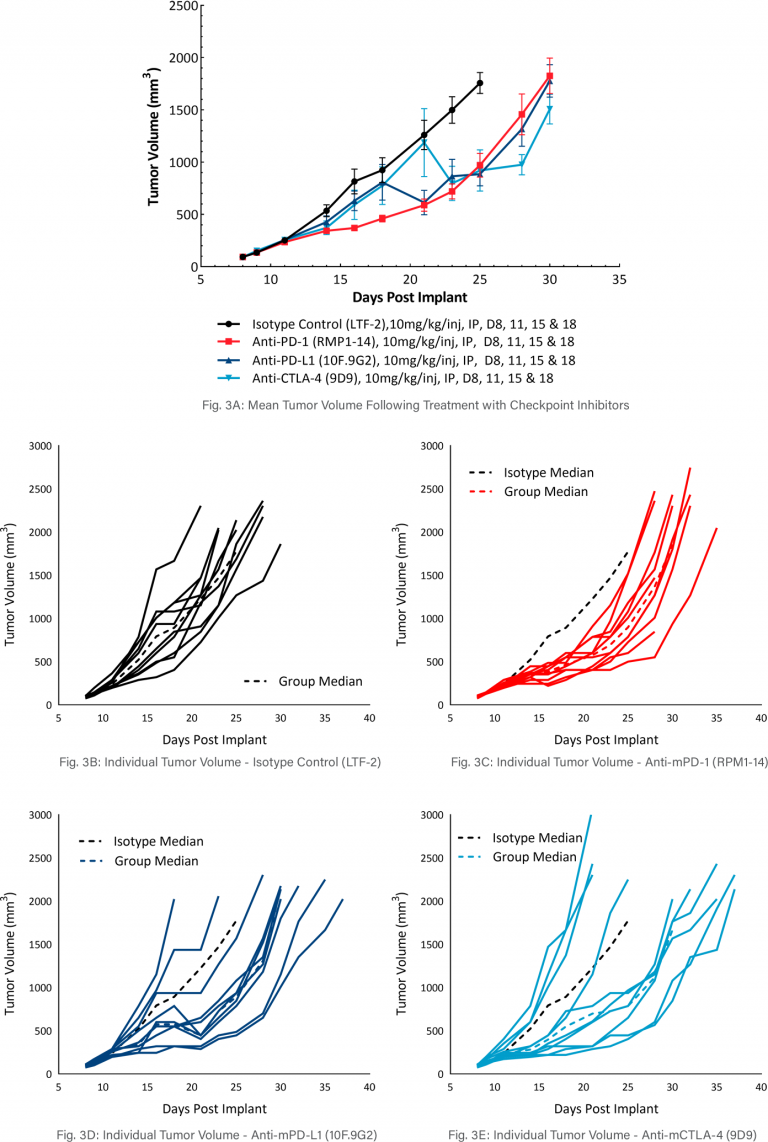
MB49 Response to Checkpoint Inhibitor Therapy
The model was evaluated for response to commonly utilized checkpoint inhibitor antibodies, anti-mCTLA-4, anti-mPD-L1, or anti-mPD-1 (Figure 3). Dosing with all test agents began once tumors were established (~100mm3). Treatment with anti-mPD-L1, anti-mPD-1, and anti-mCTLA-4 demonstrated similar, mild anti-tumor activities, with approximately 2.5-days tumor growth delay compared to the isotype control group. However, treatment with anti-mPD-L1, anti-mPD-1 and anti-mCTLA-4 did produce 20%, 20%, and 40% putative responders, respectively. The clear effect of these treatments can allow for additive or synergistic improvement in combination with candidate molecules.
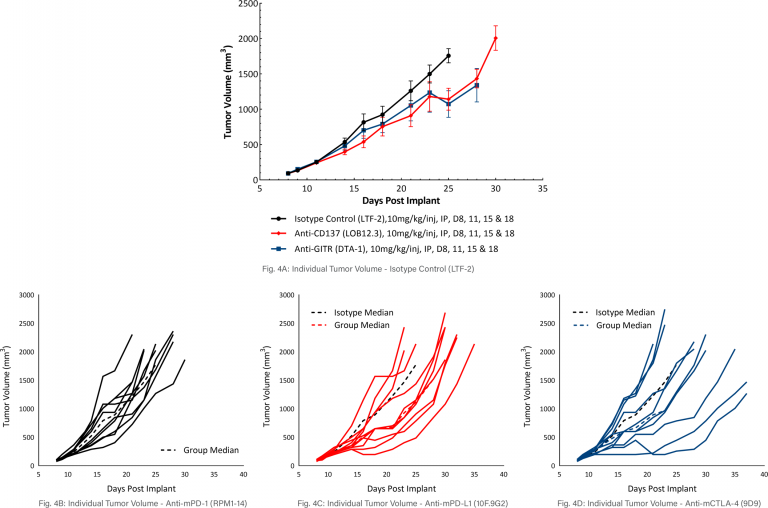
MB49 Response to Costimulatory Antibody Therapy
The response of MB49 to the costimulatory molecules anti-mCD137 and anti-mGITR was also evaluated and found to have a similar level of activity when compared to responses illustrated in Figure 3 (see Figure 4A-D). Anti-mCD137 treatment produced mild anti-tumor activity with a 3.5 day tumor growth delay and 20% putative responders. Treatment with anti-mGITR had the least amount of anti-tumor activity with a tumor growth delay of 0.9 days and 20% putative responders. However, the limited activity provides significant room for improvement with combination therapy.
The MB49 murine bladder carcinoma model can be employed as a robust preclinical immuno-oncology model. Our data supports the use of this tool in investigating novel treatment combinations with checkpoint inhibitors or costimulatory molecules.
Please contact Labcorp to speak with our scientists about how MB49, or one of our other syngeneic models, can be used for your next immuno-oncology study.
