LabCorp launches return to work services for employers
Employers Now Have Access to LabCorp’s High-Quality COVID-19 Testing Capabilities Including Its New Fingerstick Antibody Blood Test
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20200514005433/en/
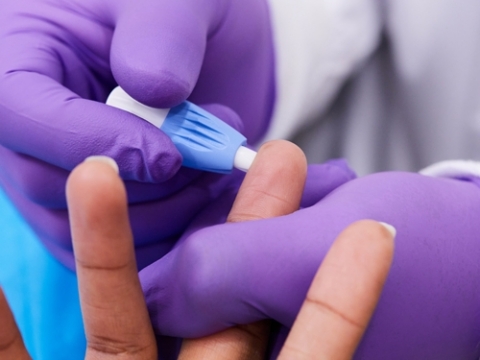
Fingerstick for COVID-19 IgG antibody test (Photo:
“We have been helping keep people safe and healthy for 50 years and are uniquely positioned to assist employers in their return to work strategies,” Dr.
LabCorp’s new convenient fingerstick antibody blood test detects IgG antibodies to the virus that causes COVID-19 and provides highly specific results equivalent to LabCorp’s other antibody blood testing methods. Fingerstick IgG antibody testing provides a convenient way to test large numbers of employees as part of return to work programs.
LabCorp’s fingerstick, or dried blood spot, IgG antibody test is being provided as a laboratory developed test, and uses the EUROIMMUN test which received Emergency Use Authorization by the
LabCorp Employer Services was previously called Wellness Corporate Solutions. In addition to LabCorp’s COVID-19 testing and flu vaccination services, LabCorp Employer Services offers comprehensive wellness solutions, including biometric screening, occupational testing, and diagnostic services. For more information, visit LabCorp’s Employer Services COVID-19 page.
About
To learn more about
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements, including but not limited to statements with respect to clinical laboratory testing, the potential benefits of COVID-19 serological testing, our responses to and the expected future impacts of the COVID-19 pandemic, and the opportunities for future growth. Each of the forward-looking statements is subject to change based on various important factors, many of which are beyond the Company’s control, including without limitation, whether our response to the COVID-19 pandemic will prove effective, the impact of the COVID-19 pandemic on our business and financial condition, as well as on general economic, business, and market conditions, competitive actions and other unforeseen changes and general uncertainties in the marketplace, changes in government regulations, including healthcare reform, customer purchasing decisions, including changes in payer regulations or policies, other adverse actions of governmental and third-party payers, the Company’s satisfaction of regulatory and other requirements, patient safety issues, changes in testing guidelines or recommendations, federal, state, and local governmental responses to the COVID-19 pandemic, adverse results in material litigation matters, failure to maintain or develop customer relationships, our ability to develop or acquire new products and adapt to technological changes, failure in information technology, systems or data security, and employee relations. These factors, in some cases, have affected and in the future (together with other factors) could affect the Company’s ability to implement the Company’s business strategy and actual results could differ materially from those suggested by these forward-looking statements. As a result, readers are cautioned not to place undue reliance on any of our forward-looking statements. The Company has no obligation to provide any updates to these forward-looking statements even if its expectations change. All forward-looking statements are expressly qualified in their entirety by this cautionary statement. Further information on potential factors, risks and uncertainties that could affect operating and financial results is included in the Company’s most recent Annual Report on Form 10-K and subsequent Forms 10-Q, including in each case under the heading RISK FACTORS, and in the Company’s other filings with the
# # #
View source version on businesswire.com: https://www.businesswire.com/news/home/20200514005433/en/
LabCorp Contacts:
Media:
[email protected]
Investors:
[email protected]
Source:


