Poster
HT-29 as a preclinical model for colorectal cancer
Treatment options for CRC are highly dependent on the stage of disease, but many patients receive cocktails of chemotherapies and in some cases radiation treatment along with chemotherapy. A number of preclinical xenograft models exist that allow pharmaceutical and biotech companies to investigate new treatment approaches for CRC. We have a number of CRC models (see Table 1) and HT-29 represents one of our most highly utilized lines. Figure 1 illustrates growth of the line following subcutaneous implant in nude mice. Most studies will stage within two weeks following implant and the tumor doubles roughly every six to seven days. We have used this model to test a number of chemotherapies, as shown in Figures 2-4.
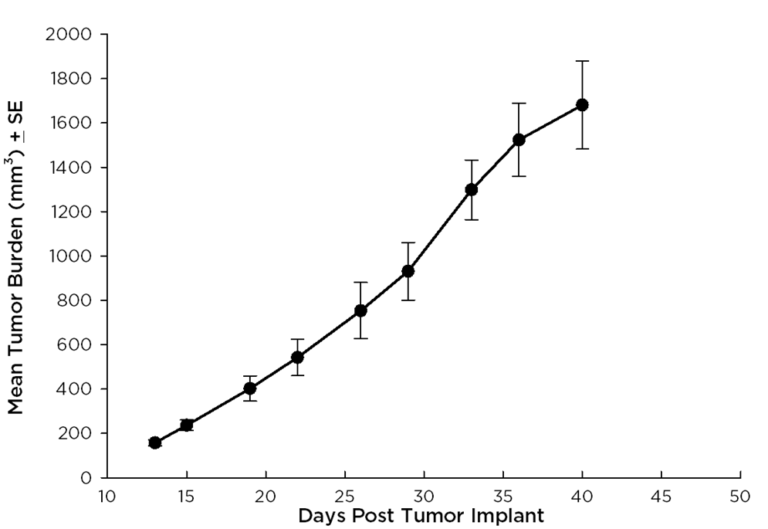
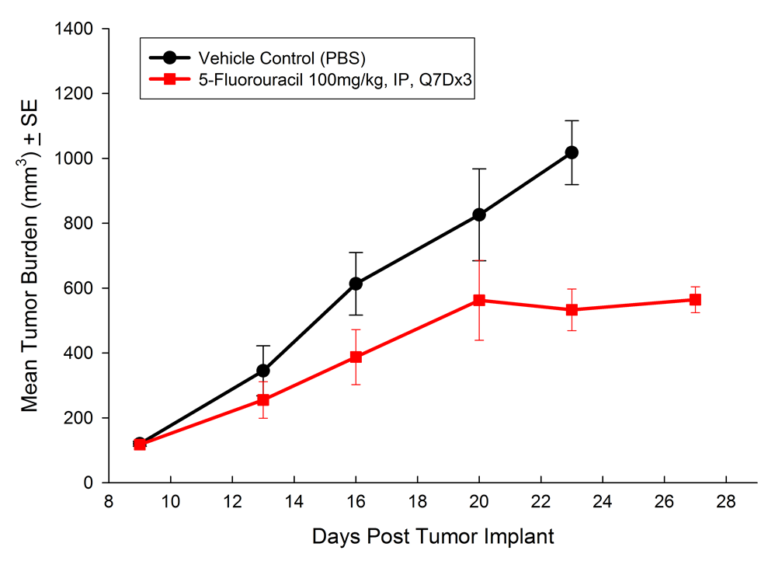
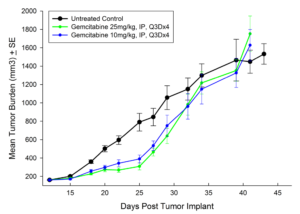
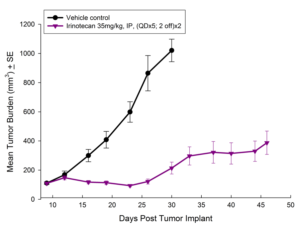
Fig. 4: Subcutaneous Growth of HT-29 Following Treatment with Irinotecan
In this model we have also evaluated the combination of radiation and gemcitabine (Figure 5) and illustrate the added benefit of the combination over either single agent treatment.
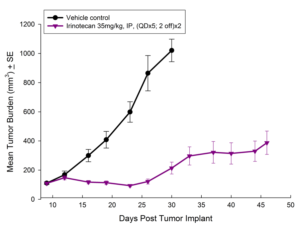
Fig. 5: Subcutaneous Growth of HT-29 Following Combination Treatment with Localized Radiation and Gemcitabine


