During preclinical research and development, CAR (chimeric antigen receptor) T-cell therapies are often investigated using a mouse as the test subject. In this setting, immunocompromised mice carry a tumor burden of human origin and are dosed with allogeneic human CAR T cells. The adoptively transferred cells are directed by the engineered chimeric antigen receptor to bind to a surface antigen on a tumor cell (CD19 for example).
As a result of the engineered T cell binding to the tumor cell, the T cell becomes activated and kills tumor cells in an MHC unrestricted manor. Mice in preclinical research studies are followed for a number of predefined parameters, evaluating tumor growth and burden to determine the efficacy of the CAR T cells (Learn more about how efficacy can be determined by bioluminescence imaging (BLI).
A key success factor of the CAR T cell antitumor response is how long the CAR T cells exist after infusion into the host (persistence). Despite full functionality, it has been shown that poor persistence of CAR T cells can limit an effective antitumor response.1 In the clinical setting, long-term remission in patients with hematological malignancies is associated with sustained persistence of CAR T cells.2 Co-stimulatory signaling is thought to influence T cell expansion and persistence. Preclinical studies investigating the efficacy of CAR constructs that are engineered to include the 4-1BB co-stimulator domain have been associated with long CAR T cell persistence, a slower and sustained effector function, and a higher proportion of memory T cells.3 Assessing CAR T persistence in preclinical studies have implications in determining clinical success.
This technology spotlight will provide the reader with information about measuring CAR T cell persistence, using flow cytometry during the preclinical phase.
Mouse Models for CAR T Cell Candidate Evaluation
In September of 2017 the Food and Drug Administration approved the first CAR T cell therapy. The cell therapy is marketed by Novartis and is called Kymriah. Kymriah is also known as tisagenlecleucel and was approved for children and young adults who no longer respond to standard therapies for B cell acute lymphoblastic leukemia. Kymriah is based on the work of Carl June, PhD., at the University of Pennsylvania in NSGTM mice.
The NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mouse, commonly known by the branded name, NOD scid gamma (NSG™), do not express the Prkdc gene nor the X-linked Il2rg gene. The scid (severe combined immune deficiency) mutation is in the DNA repair complex protein Prkdc and renders the mice B and T cell deficient. These mice do not have complement nor natural killer (NK) cells and are deficient in cytokine signaling pathways. These deficiencies make the mice ideal and highly amenable to engraftment of human immune cells, including CAR T cells. The mice engraft a wide range of human tumors and are the gold standard for preclinical CAR T cell research.
In our Preclinical Oncology department, we can use any commercially available mouse strain for CAR T cell therapy and other adoptive cell transfer studies, including NSGTM mice and, under certain conditions, we can accept specialty mice as well.
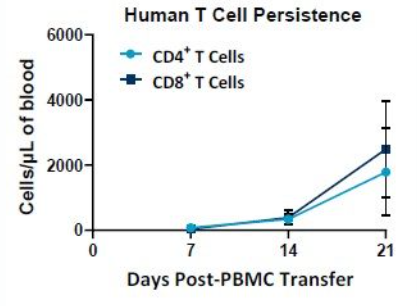
The PersistenceTTM Panel from Preclinical Oncology is used to generate longitudinal CAR T cell persistence data can be used with as little as 5 uL of whole blood. It is formatted as a lyse/no wash assay with absolute counts. The panel has flexibility, offering the opportunity to add specific anti-CAR probes in the FITC, PE and APC channels. NSG mice from the Jackson Laboratory (Bar Harbor, Maine, USA) were administered 2x107 human PBMCs, whole blood was drawn 7-, 14- and 21-days post administration and subjected to the PersistenceTTM Panel evaluation.
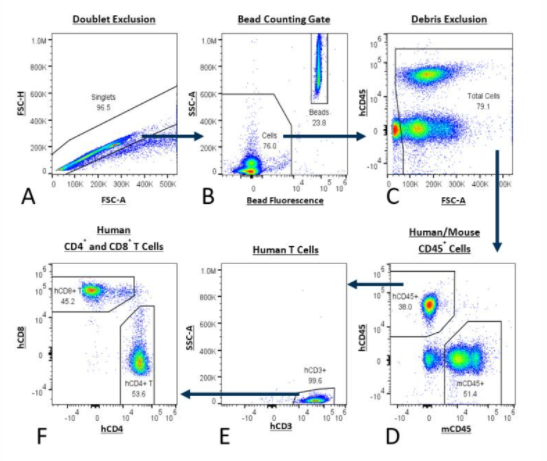
Table 1 describes the reagents used in the PersistenceTTM Panel. The gating strategy is detailed in Figure 1.
Antibody/Dye | Description |
|---|---|
mCD45 | Mouse pan-hematopoietic cells |
hCD45 | Human pan-hematopoietic cells |
hCD3 | Human pan-T cell marker |
hCD4 | Human CD4+ T cell marker |
hCD8 | Human CD8+ T cell marker |
Counting Beads | Provides absolute counts |
Viability Dye | Dead cell exclusion |
Optional: CAR Specific | FITC, PE or APC |
Table 1: PersistenceTTM Panel of antibodies and description of their use.