Capabilities
Interactions between potential therapeutics and the immune system are broadly split into two categories:
Immunology and Immunotoxicology supports large molecule, small molecule, and advanced therapy safety assessment
An integrated program with deep scientific and technical expertise at 7 global sites
High quality studies delivered on time at an affordable price
Interactions between potential therapeutics and the immune system are broadly split into two categories:
As your molecule moves through the drug discovery process, we use information and insights gained through immunology and immunotoxicology testing to support and guide your program.
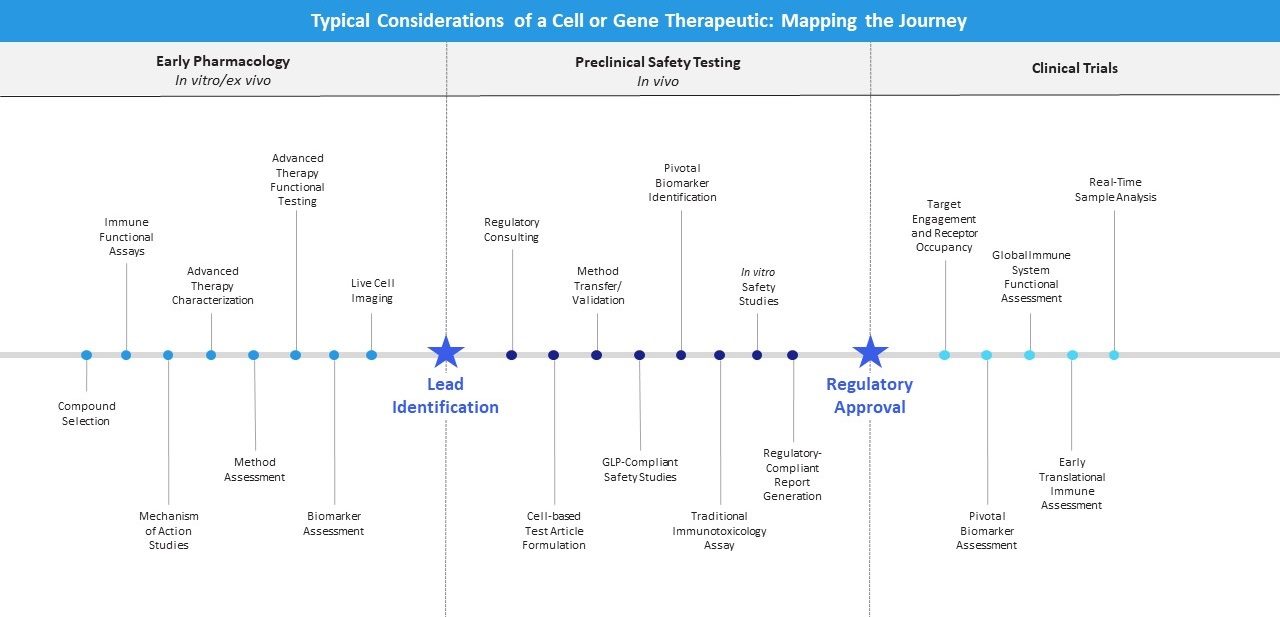
To guarantee success you need a partner with outstanding scientific acumen, insight into the regulatory environment, and the ability to employ state-of-the-art technology. Designing a package of studies to investigate the preclinical pharmacology specific to your compound, we can direct and guide your regulatory-compliant program, or simply act as an experienced and accredited lab to run your trial. Select the endpoints relevant to your goals, and take your molecule to the next level.
With ever evolving innovation in the biotech and pharmaceutical industries, we know there is no such thing as a one-size-fits-all approach. Our focus is building an immunology and immunotoxicology service around you – matched to the specific needs of your company and your molecule. Labcorp Drug Development works across the full spectrum of companies from start-up biotech firms, using our immunological and regulatory knowledge to give direction, through to large, established pharmaceutical firms where we can act as a GLP-compliant partner who can run your assays effectively and efficiently.
Our experts drive your I&I program, employing the right technology and assays to deliver on selected endpoints and answer your key questions.
Both predictive and diagnostic, identifying biomarkers early is crucial to the success of your molecule. With insights from across Labcorp Drug Development, we can support you seamlessly through preclinical testing to clinical laboratory services where your biomarkers can make the biggest impact.
Supporting the discovery and lead hit identification phases of your development program.
We can design a program, creating specific reagents and building assays, to find the information you need.
Supporting your GLP-compliant toxicology studies for IND filing.
We work with you to define how thorough and in-depth this process needs to be, based on a weight of evidence approach.
Biomarkers come in all shapes and sizes and at Labcorp Drug Development, we use an equally variable approach to assess biomarkers that are right for your molecule, at every stage of your drug discovery journey.
We support you in identifying when to use GLP and non-GLP assays, ensuring your program is cost-effective and fit-for-purpose. Our focus is then getting you the data you need to support a successful IND/CTA filing, getting you to FIH and beyond. We build our program around your molecule, anticipating regulatory challenges and taking a strategic approach that leverages state-of-the-art technology and expertise.
At Labcorp Drug Development, we provide you with evidence and data that goes beyond guidance requirements, so you have a better holistic understanding of your molecule.